Voluntary recall - ilium Meloxicam Anti-Inflammatory Oral Suspension for Dogs - 30 mL pack size
15 Nov 2024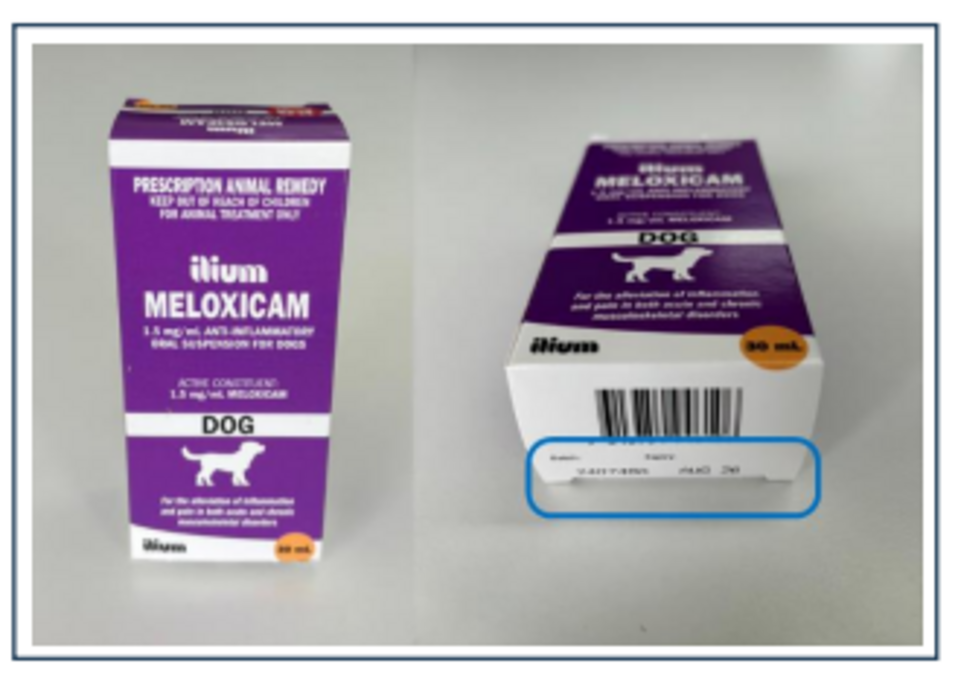
Identifying features:
Name: ilium Meloxicam Anti-Inflammatory Oral Suspension for Dogs - 30 mL pack size
APVMA Registration Number: 60023
APVMA Approved Label Number: 139996
Batch Number: 240748A
Sold by veterinary wholesalers in all states and territories between 06/09/2024 to 06/11/2024
On 06/11/2024, Troy Laboratories Pty Ltd (ABN: 000 283 769) initiated a voluntary recall under section 106 of the Agricultural and Veterinary Chemicals Code scheduled to the Agricultural and Veterinary Chemicals Code Act 1994 (Cth) in relation to the chemical product described above.
Reason for voluntary recall:
Some units in batch 240748A have an incorrect leaflet (ilium Meloxicam 0.5 anti-inflammatory oral suspension for cats) enclosed. All other aspects of the product are in order (contents, label, carton, syringe).
Hazard:
Following the dosing instructions from the incorrect leaflet (for use in cats, rather than dogs) could potentially lead to underdosing in dogs and therefore reduced efficacy. However, the information provided on both the label and carton are in order.
What to do:
Wholesalers should immediately quarantine all stock on hand of the recalled batch (240748A). Troy (details included below) will provide advice on management of affected units and arrangements for replacement. Veterinarians will be provided with replacement leaflets for affected units.
For further information:
Please direct all calls and any queries concerning this voluntary recall to Troy Laboratories Customer Service, on 02 8808 3611.
