Voluntary recall notice: ilium Meloxicam Anti-Inflammatory Oral Suspension for Cats and Dogs
08 May 2025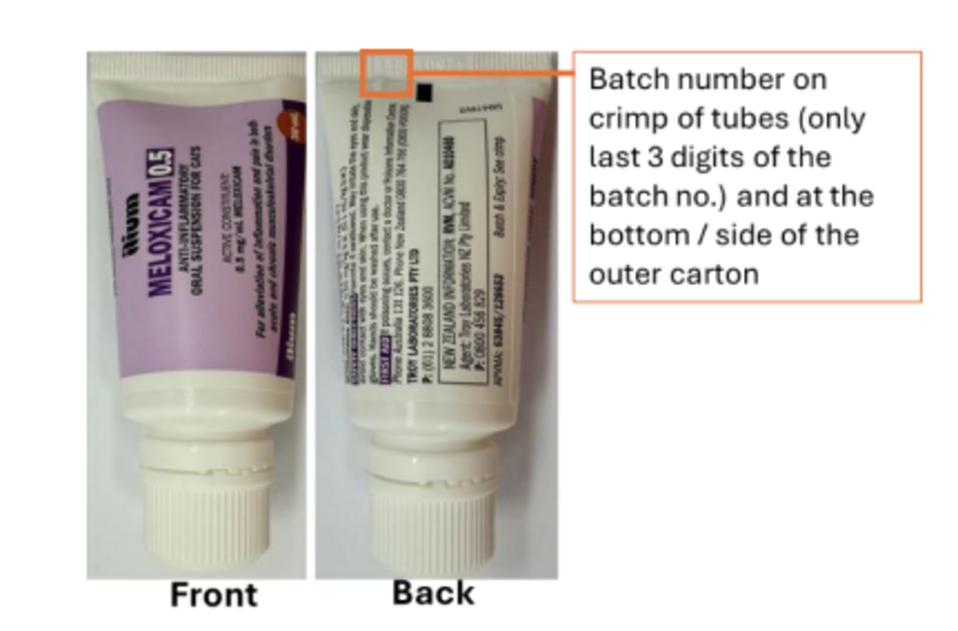
Notification to APVMA of voluntary recalls
ilium Meloxicam 0.5 Anti-Inflammatory Oral Suspension for Cats
APVMA registration number: 63845
APVMA approved label numbers: 128653 or 135342 or 136025 or 138479 or 139436
Batch numbers: All batches (Refer to Appendix A)
Sold by veterinary wholesalers in all states and territories between 19/10/2023 to 01/05/2025
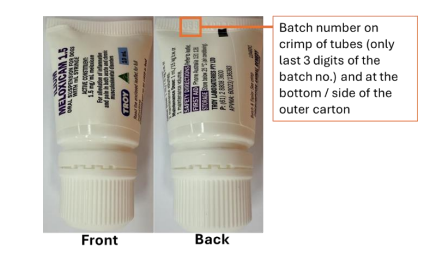
ilium Meloxicam Anti-Inflammatory Oral Suspension for Dogs
APVMA registration number: 60023
APVMA approved label numbers:135339 or 136383 or 138430 or 139996
Batch numbers: All batches (Refer to Appendix A)
Sold by veterinary wholesalers in all states and territories between 08/06/2023 to 01/05/2025
On 01/05/2025, Troy Laboratories Pty Ltd (ABN: 000 283 769) initiated a voluntary recall under section 106 of the Agricultural and Veterinary Chemicals Code scheduled to the Agricultural and Veterinary Chemicals Code Act 1994 (Cth) in relation to the chemical product described above.
Reason for voluntary recall:
Some units of only the 10- and 30-mL pack sizes, still within the expiry date, may be leaking.
Hazard:
Leaks can lead to wastage, contamination, or a reduced amount of the product available for use. If the tube is not leaking, there is no risk, and the product does not need to be returned.
What to do:
Please isolate and place in quarantine, any leaking units of 10- and 30-mL pack sizes of the product. Troy (contact details included below) will provide advice on management of affected units and arrangements for replacement.
For further information
Please direct all calls and any queries concerning this voluntary recall to Troy Laboratories Customer Service, on 02 8808 3670.
